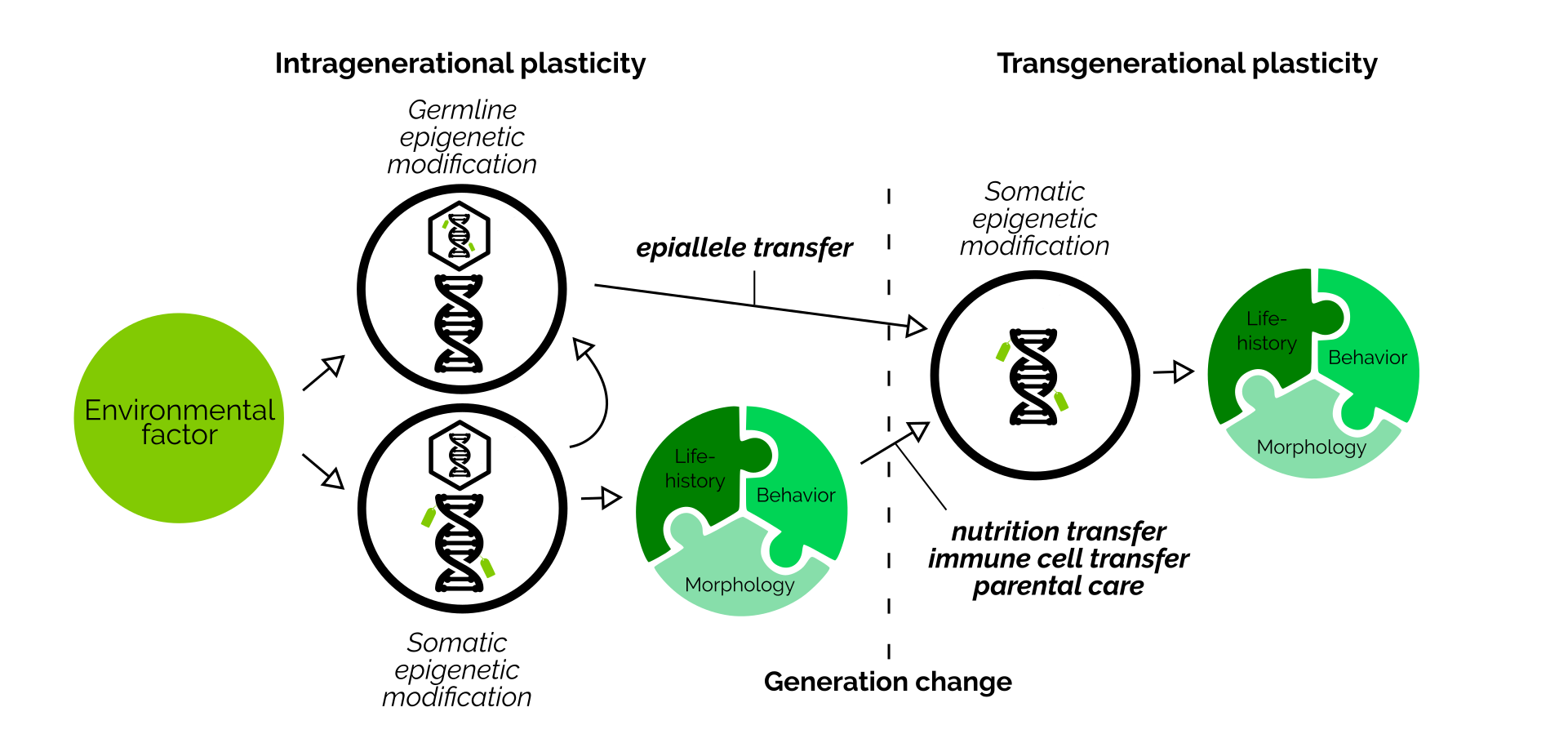
Transgenerational plasticity is a useful ability. To enable this process, parents collect information about their environment during their lifetime, and then pass this information down to offspring. This information then allows young animals to prepare themselves ideally to live in the same environment.
Over the last decades, many different ways how information can be transmitted across generations have been identified. For example, both mothers and fathers can transmit epigenetic marks (epialleles) together with their DNA within sperm and eggs. Mothers can also embed hormones within the eggs. Fathers can alter their seminal fluid. Furthermore, parents can communicate information to eggs by handling them differently during parental care. And lastly, offspring can also perceive their environment themselves.
Now, which information is more important? This has been a long unresolved question that I wanted to tackle in my research. For this purpose, I conducted a large experiment with the model system Pimephales promelas that forms shoals as a distinct social behavior. Under high predation risk, shoals are dense so that individuals are not getting eaten. However, as in other social animals, forming dense shoals also has disadvantages as it leads to more competition, less food and to greater transmission risk of pathogens – doing so is clearly not always optimal. Hence, like other animals these fish are known to adjust their shoaling density dependent on the level of perceived risk.
This trade-off between the costs and benefits of antipredator responses is what leads to a hypothesis developed by Lima and Bednekoff in 1999. This theory is called the risk allocation hypothesis. It predicts that when predation risk is constantly high, it is beneficial for individuals to respond with clear antipredator defenses only during immediate predator attacks, and not all the time as this would not allow individuals to gather enough food. In contrast, when predation risk is usually low, and individuals can forage all the time, which allows them to be full, it is better to respond to sudden predation events with clear, long-term antipredator responses. In a shoaling context, that means that when predation risk is believed to be high, shoals should be less responsive to a single risky event, but the opposite pattern should emerge in low-risk environments.
I exposed mothers, fathers, males that provided parental care (in this species parental care is solely provided by fathers) and offspring themselves to either high predation risk as communicated through exposure to alarm cues – or to low predation risk as simulated by exposure to tap water. Some of these treatments involved parental care while offspring were still inside eggs, others did not. By combining these treatments, I reached 12 different combinations and tested shoaling behavior in 2810 one-month old offspring. For this purpose, I assessed how close offspring swam to each other before and after a simulated predator attack.
Interestingly, treatments that involved parental care consistently caused offspring to form densest and least dense shoals, respectively. When the caring individual did not experience risk, offspring shoals were loose and were very sensitive to the simulated predator attack. However, when the male that provided care had perceived high predation risk prior to the care period, offspring formed dense shoals and were less sensitive to the simulated predator attack.
Without parental care, maternal experience with high risk was more important than paternal experience, but offspring responded most appropriately when both parents were exposed to either high or low risk. The risk information that offspring assessed themselves was only important when no risk information was transmitted from parents (i.e., when parents were never exposed to risk).
Taken together, this research highlights the high relative importance of parental care during the embryonal period (while offspring are still within their eggs) for the formation of optimal social behavior in the next generation. Many studies on transgenerational plasticity explicitly exclude parental care from their experimental design, and the results here show that this practice may lead to incorrect consequences regarding the relevance of transgenerational plasticity in allowing animals to cope with environmental change.
This study was published in Open Access format at BMC Ecology and Evolution. That means, it is freely available to everyone, and you are welcome to take a peek if you are interested in reading more details. It is the first transgenerational plasticity study that has arisen from my stay at the University of Saskatchewan, Canada – and more are to come.